DTRAX® is distributed in Germany exclusively by FEH Medizintechnik GmbH.
DTRAX®
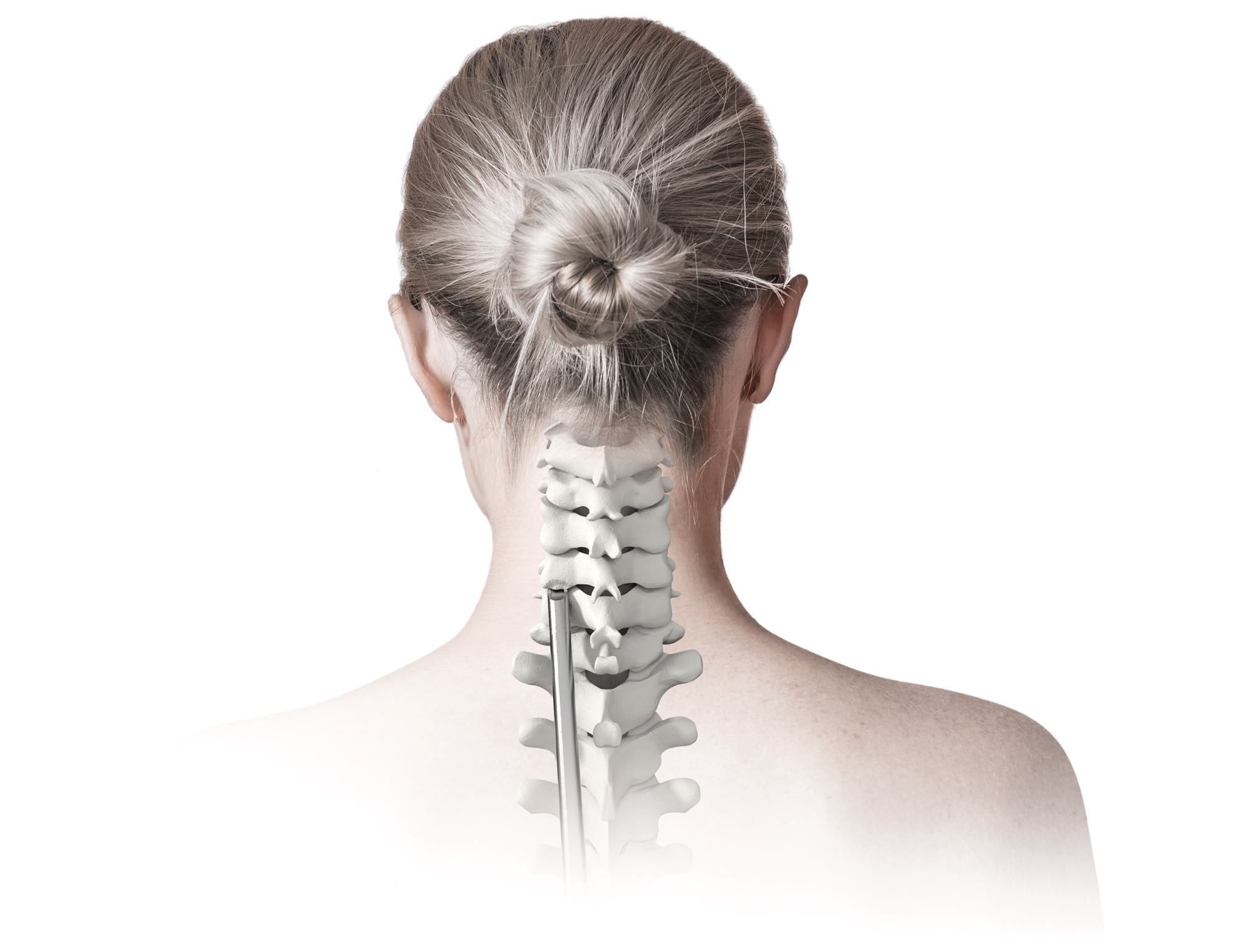
Advancing Posterior Cervical Fusion
Safe, Effective, Tissue-Sparing.
Multiple clinical publications on Posterior Cervical Fusion with DTRAX® demonstrate that the procedure is safe and effective at relieving neck and arm pain associated with cervical radiculopathy.
An Innovative Approach
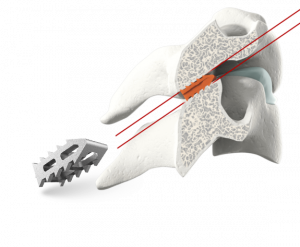
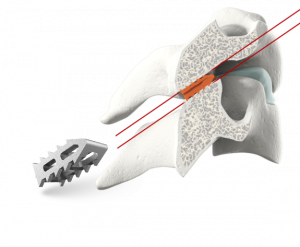
A Closer Look
Benefits of Posterior Cervical Fusion with DTRAX® may include:
Benefits of Posterior Cervical Fusion with DTRAX® may include:
Indications
The GL-DTRAX® instruments, cages, and bone screws are indicated for use in skeletally mature patients for posterior cervical treatment at C3–C7 (inclusive) spinal levels for patients with single level radiculopathy due to degenerative disc disease (DDD) as defined by back pain of discogenic origin with degeneration of the disc confirmed by history and radiographic studies and/or degenerative disease of the facets.
Providence’s family of products offers a variety of surgical options to treat cervical radiculopathy. Patients should always consult their physician to determine which course of treatment is most appropriate.
Kontraindikationen:
Die posteriore zervikale Fusion mit DTRAX® darf bei Vorliegen jeglicher der folgenden Umstände nicht verwendet werden:
REQUEST MORE INFORMATION
“*” indicates required fields. You can also find out how carefully we handle your data in our Privacy Policy regulations.